🌟 Calcium Carbonate – Earth's Universal Sculpture in Stone!From towering mountains to glittering caves, Calcium Carbonate (CaCO₃) is nature’s architect—an elegant, crystalline expression of the Earth’s cycles, built over time through ocean life, geologic pressure, and chemical balance. More than just white rock, it’s a testament to ancient seas, biomineralization, and sedimentary art.
📌 Basic Identification
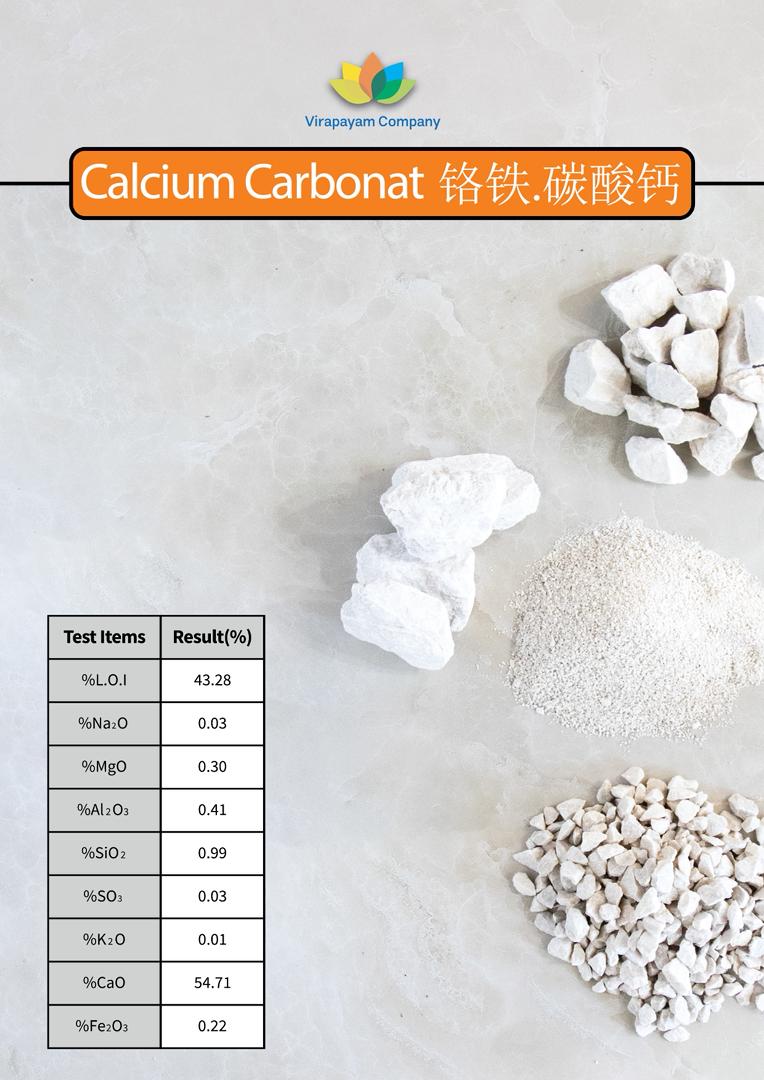
Name: Calcium Carbonate
Chemical Formula: CaCO₃
Mineral Forms: Calcite, Aragonite, and Vaterite (polymorphs)
Mineral Group: Carbonates
Crystal System:
- Calcite: Trigonal
- Aragonite: Orthorhombic
Mohs Hardness:
- Calcite: 3
- Aragonite: 3.5–4
Specific Gravity: ~2.7 (Calcite), ~2.9 (Aragonite)
Luster: Vitreous to pearly
Transparency: Transparent to translucent
Streak: White
Cleavage: Perfect in three directions (especially in calcite)
Fracture: Conchoidal to uneven
Color: White, colorless, or tinted shades of gray, yellow, pink, and blue
🌍 Geological Formation and Origin
Calcium Carbonate is formed through both biological and inorganic processes:
🧬 Modes of Formation:
Marine Biogenic Deposition: Microscopic organisms like foraminifera, corals, and algae produce CaCO₃ as shells or skeletons, which accumulate as limestone or chalk.
Precipitation in Caves: Dripping groundwater forms stalactites and stalagmites through slow evaporation of carbonate-rich water (travertine and speleothems).
Metamorphism: Under heat and pressure, limestone recrystallizes into marble, a dense, interlocking form of calcite.
🌊 Environments:
Shallow marine shelves
Coral reefs
Karst landscapes
Hot spring deposits
Sedimentary basins
Deep ocean calcareous oozes
🌎 Global Occurrence and Deposits
Calcium Carbonate is one of the most abundant minerals on Earth.
Major Deposits:
Iran: Rich in marble and limestone across Fars, Isfahan, and Lorestan
Italy: Carrara – world-renowned white marble
USA: Appalachian region (limestone belts)
Germany: Swabian Jura – Jurassic marine limestones
United Kingdom: White Cliffs of Dover (chalk)
Egypt: Eocene limestone of the Mokattam Hills
China, India, Turkey: Significant deposits across geological zones
Forms Found:
Thick limestone beds
Marble quarries in metamorphic zones
Chalk cliffs and sedimentary plains
Travertine terraces and cave formations
🔬 Mineralogical and Physical Characteristics
Crystal Habit
- Calcite: Rhombohedral, scalenohedral (“dogtooth”), or massive granular
- Aragonite: Needle-like or radiating columnar forms
Reactivity
- Readily effervesces (fizzes) with dilute hydrochloric acid – a classic diagnostic test
Optical Properties
- Calcite displays strong birefringence – splitting of light into two rays
- Refractive Index: ~1.48–1.65 depending on polymorph
Color and Appearance
- Typically colorless to white, but can be tinted by iron, copper, or organic material
🧪 Chemical Properties and Stability
Stable at surface conditions, but breaks down under acidic environments
Soluble in weak acids (HCl, CO₂-rich water), forming calcium bicarbonate
Prone to diagenetic recrystallization in sedimentary basins
High-temperature stability makes marble a common metamorphic product
🧭 Environmental and Geological Significance
Carbon Cycle Anchor: Plays a major role in global carbon storage and ocean chemistry
Climate Proxy: Isotopic composition (δ¹³C, δ¹⁸O) in carbonates is used to reconstruct past climates
Fossil Record Host: Limestone often contains well-preserved fossils
Karst Landscapes: Forms unique terrain including sinkholes, caves, and underground rivers
🛑 Handling and Preservation
Sensitive to acidic conditions and high humidity
Avoid acid contact or prolonged exposure to moisture
Store in cool, dry conditions if samples are to be preserved for optical or geochemical study
Polished forms (e.g., marble) should be kept free of abrasives and sealed periodically
✅ Summary
Calcium Carbonate is a geologic chameleon—able to build reefs, form mountains, and etch masterpieces in stone. Whether as glittering calcite crystals in a cave or sweeping white cliffs facing the sea, it embodies both elegance and elemental science. Through countless forms and environments, it silently chronicles the Earth’s geochemical rhythms and ancient life.
💎 Calcium Carbonate – Earth’s eternal sculptor, in crystal and cliff.

